Medical Freedom Act (MFA) is based on real world historical events. This button shows you historical news articles and videos that are related to part of the MFA. This lets you see for yourself why MFA is needed urgently.
6.3.1. All medical treatments, procedures, and products shall be assigned safety ratings for specific safety envelopes. A safety envelope is a known quantity derived from actual data. The safety envelope is constituted of a set of tested domains defined by any scientific parameter.
6.3.2. Safety envelopes shall be assigned regardless of the phase of trial, conditional approval, emergency use authorization, or full authorization status.
6.3.3. The designations “safe” or “possibly safe” shall be apply only to a medical treatment, procedure, or product safety envelope. There is no limit to the number of safety envelopes with corresponding ratings. Clinical trials and experiments shall also employ safety envelopes except that these envelopes will expectedly evolve as subject data is observed. Once a safety envelope for a trial is defined, its parameters shall not be altered. Only the outcomes of adverse events and fatalities will change. A new safety envelope will be defined if any parameter is changed and a new trial or experiment is necessary.
6.3.4. Safety envelope parameters shall include, but are not limited to:
6.3.4.1. Age,
6.3.4.2. Sex,
6.3.4.3. Weight or Body Mass Index,
6.3.4.4. Pre-existing conditions,
6.3.4.5. Exposure to previous contagions,
6.3.4.6. Genetic predispositions,
6.3.4.7. Drug interactions,
6.3.4.8. Vaccination history,
6.3.4.9. Probability of exposure to related or target contagions.
6.3.5. A medical treatment, product, or procedure shall not be considered to have a safety rating applicable for a person if the person does not meet all the parameter criteria of the safety envelope.
6.3.6. Legacy medical treatments, procedures, or products will not be assigned safety ratings unless they are
6.3.7. Personally identifiable information.
6.3.7.1. For the purposes of this section, the following shall be redacted to meet the requirements of public disclosure under this section:
6.3.7.1.1. Name
6.3.7.1.2. Social Security Number
6.4. [RATINGS] Safety Ratings
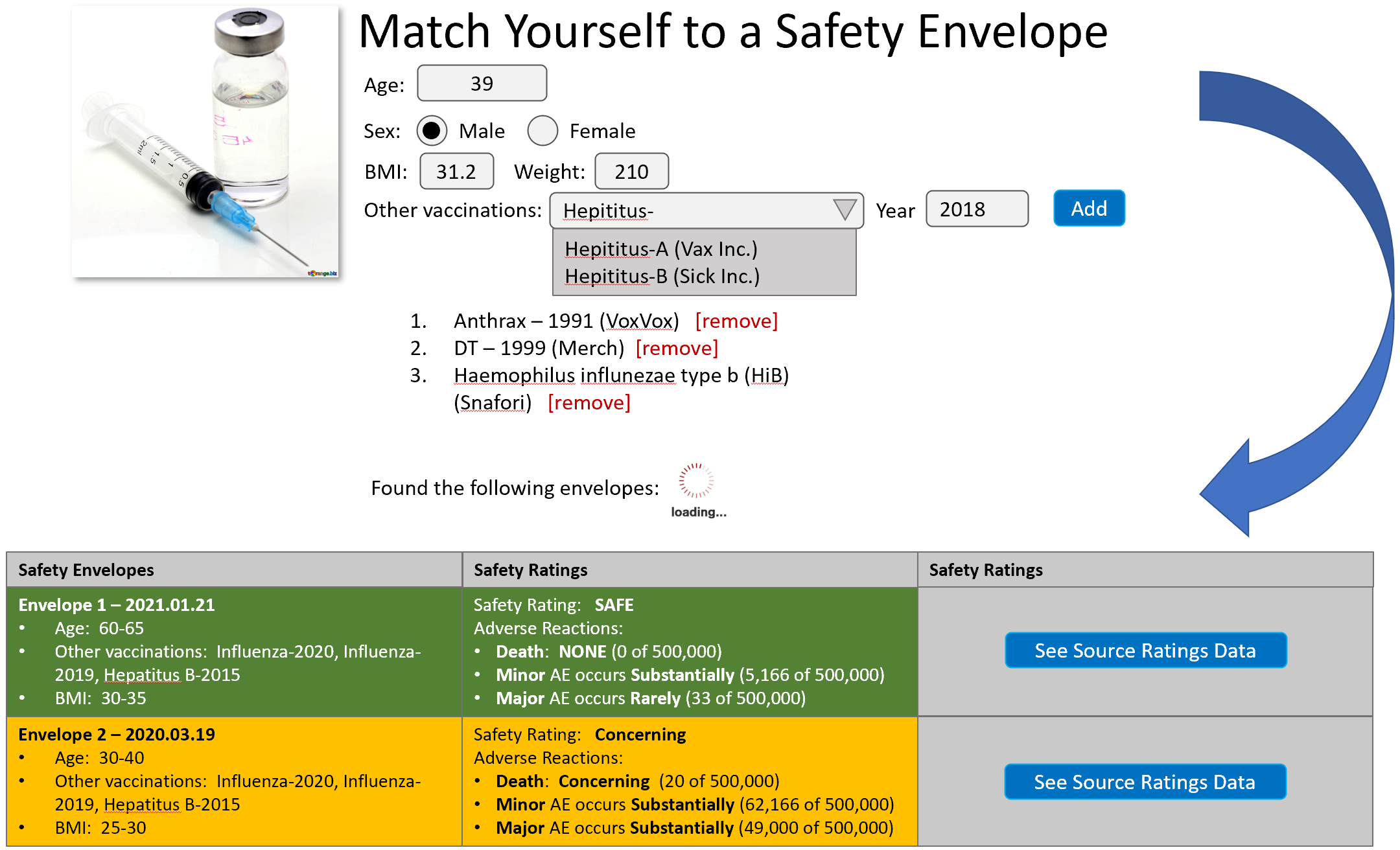
6.4.1. A safety rating is an aid to informed consent and is not intended to stifle research or trials. A safety rating does not limit trials, emergency use authorization, or full authorization of a medical treatment, procedure, or product. A safety rating is a metric based on absolute values and does not scale according to the number of people treated.
6.4.1.1. A treatment may be defined as a single product or the use of various products or procedures prescribed by a protocol.
6.4.2. Safety ratings may originate from any medical practitioner or scientific source. No formal requirements shall be needed but no anonymous sources shall exist.
6.4.2.1. The originator(s) must provide their full name(s), medical or scientific credentials, and disclose any conflicts of interest such as, but not limited to, funding sources, corporate board memberships, being a shareholder of an involved or related corporation, earning royalties for research, or having any relationship with a person in an involved entity.
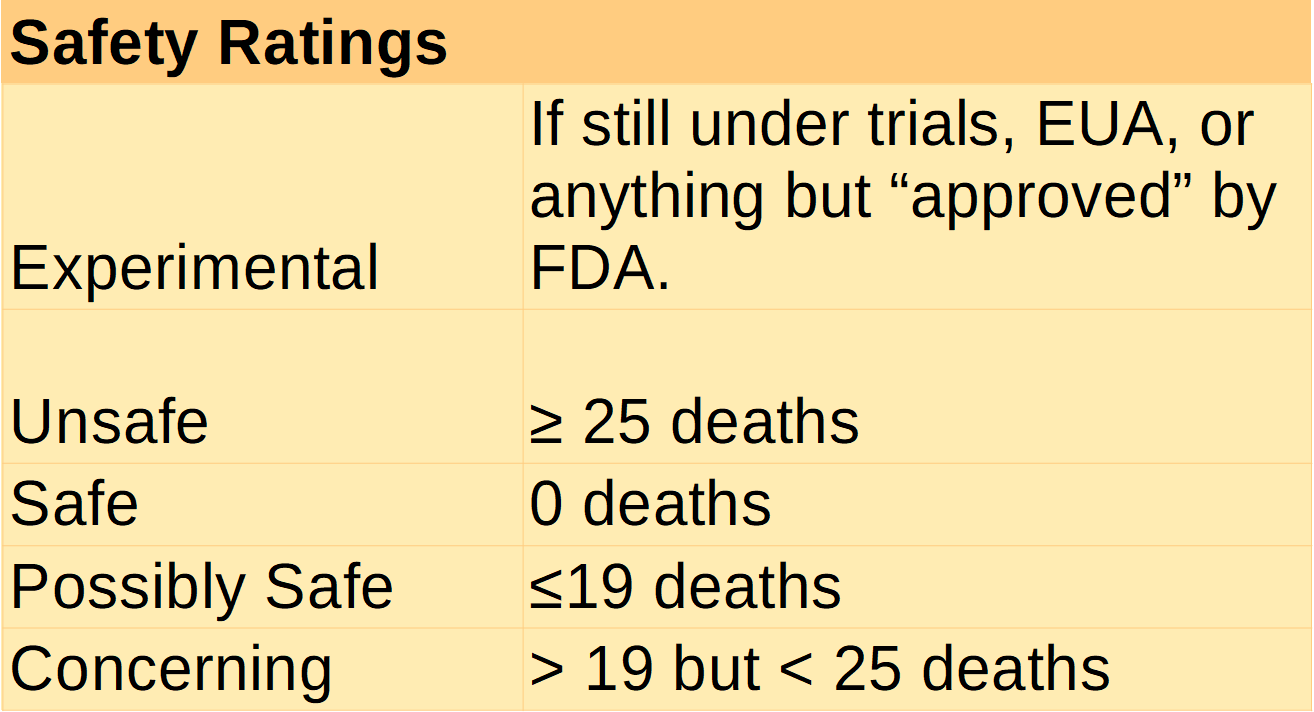
6.4.3. Safe
6.4.3.1. A medical treatment, procedure, or product is considered safe only when:
6.4.3.1.1. Not a single person has been severely harmed, fatally harmed, or permanently harmed from its use.
6.4.3.2. A treatment or medical product is eligible to be considered “safe” once at least 10 years has elapsed since the last severe or fatal event occurred.
6.4.3.3. A medical product, treatment, or procedure is not eligible to gain safety credit towards the 10 year requirement if the product is not on the commercial market or reasonably available for a continuous 10 year period.
6.4.3.4. Any medical treatment, procedure, or product comprehensively regarded as safe may not have any safety envelopes evaluated as possibly safe or experimental. Individual safety envelopes may have any safety rating according to the criteria of this Act.
6.4.3.5. No medical treatment, product, or procedure shall be considered safe either comprehensively or by safety envelope if it benefits a person by injuring others.
6.4.3.6. No entity shall qualify a medical treatment, procedure, or product unless all clinical trials have fully completed and the FDA has “approved” it with applicable safety envelope(s).
6.4.4. Possibly Safe
6.4.4.1. A medical treatment, procedure, or product is considered possibly safe only when:
6.4.4.1.1. Any fatal adverse event occurs in at least more than 1 persons, but in the lesser of 10 persons or 2% of any persons using the medical treatment, procedure, or product.
6.4.4.1.2. Any permanent adverse event occurs in no more than 10 persons for every 1,000,000 treated.
6.4.4.2. Any medical treatment, procedure, or product regarded as possibly safe in any safety envelope may not be comprehensively regarded as safe. Individual safety envelopes may have any safety rating according to the criteria of this Act.
6.4.4.3. No medical treatment, product, or procedure shall be considered possibly safe either comprehensively or by safety envelope if it benefits one by injuring others.
6.4.5. Unsafe
6.4.5.1. Any medical treatment, procedure, or product which fatally injures human life in excess of 25% of the total mortality rate of the disease itself shall have a safety envelope of unsafe for treatment of that disease. This does not preclude other treatment targets from having different safety envelopes.
6.4.5.2. The comprehensive safety envelope of any medical treatment, procedure, or product shall be no higher than experimental if it contains an unsafe safety envelope for any treatment target.
6.4.6. Concerning
6.4.6.1. Any medical treatment, procedure, or product is considered experimental outside of a safety envelope which is qualified as either safe or possibly safe.
6.4.6.2. Any medical treatment, procedure, or product regarded as experimental in any safety envelope may not be comprehensively regarded as safe or possibly safe. Individual safety envelopes may have any safety rating according to the criteria of this Act.
6.4.7. Experimental
6.4.7.1. Any product which does not have at least a 10 year approved an in use history is experimental by default but experimental can be modified by another safety rating (e.g. experimental – possibly safe).
6.5. [EFFICACY] Efficacy.
6.5.1. An efficacy target is a concise definition of what the medical treatment, procedure, or product is intended to remedy.
6.5.1.1. Efficacy targets of vaccines shall always include:
6.5.1.1.1. Efficacy against prevention of transmission.
6.5.1.1.2. Efficacy against prevention of infection.
6.5.1.1.3. Efficacy against prevention of permanent injury.
6.5.1.1.4. Efficacy against prevention of death.
6.5.2. Efficacy targets shall be compared against:
6.5.2.1. Placebo.
6.5.2.2. Persons previously treated.
6.5.2.3. Competing treatments.
6.5.3. Any medical practitioner or person conducting research may provide efficacy target data based on their own experiments or research to the FDA.
6.5.4. The efficacy target of any medical treatment, procedure, or product shall be prominently displayed by any entity in any product literature, product insert, advertisement, promotional material, or any materials used in any capacity of informed consent.
6.6. [ADVERSE] Adverse Events.
6.6.1. An adverse event is any condition which is not normal for that person, occurring any time after having received a medical treatment, procedure, or product or as a third party tangentially affected by a suspected communicable phenomena.
6.6.2. Types of Adverse Events

6.6.2.1. Notwithstanding other technical or scientific definitions, the following adverse event types shall be used by any entity to provide informed consent, to educate, to inform, or to advertise a medical treatment, procedure, or product.
6.6.2.1.1. Death
6.6.2.1.1.1. Any fatal event of a person that occurs up to 5 yrs after, and is related to, the administration of a medical treatment, procedure, or product.
6.6.2.1.2. Serious
6.6.2.1.2.1. Any adverse event experienced by a person(s) as a consequence of a medical treatment, procedure, or product use that has an effect on the ability of a person to move, think, speak, be free from incapacitating pain, or perform their occupation, for a period exceeding 365 days.
6.6.2.1.3. Major
6.6.2.1.3.1. Any adverse event experienced by a person(s) as a consequence of a medical treatment, procedure, or product use that has an effect on the ability of a person to move, think, speak, be free from incapacitating pain, or perform their occupation, for a period not exceeding 365 days.
6.6.2.1.4. Minor
6.6.2.1.4.1. Any adverse event experienced by a person(s) as a consequence of a medical treatment, procedure, or product use that has no effect on the ability of a person to move, think, speak, be free from incapacitating pain, or perform their occupation, for a period up to and including 14 days.
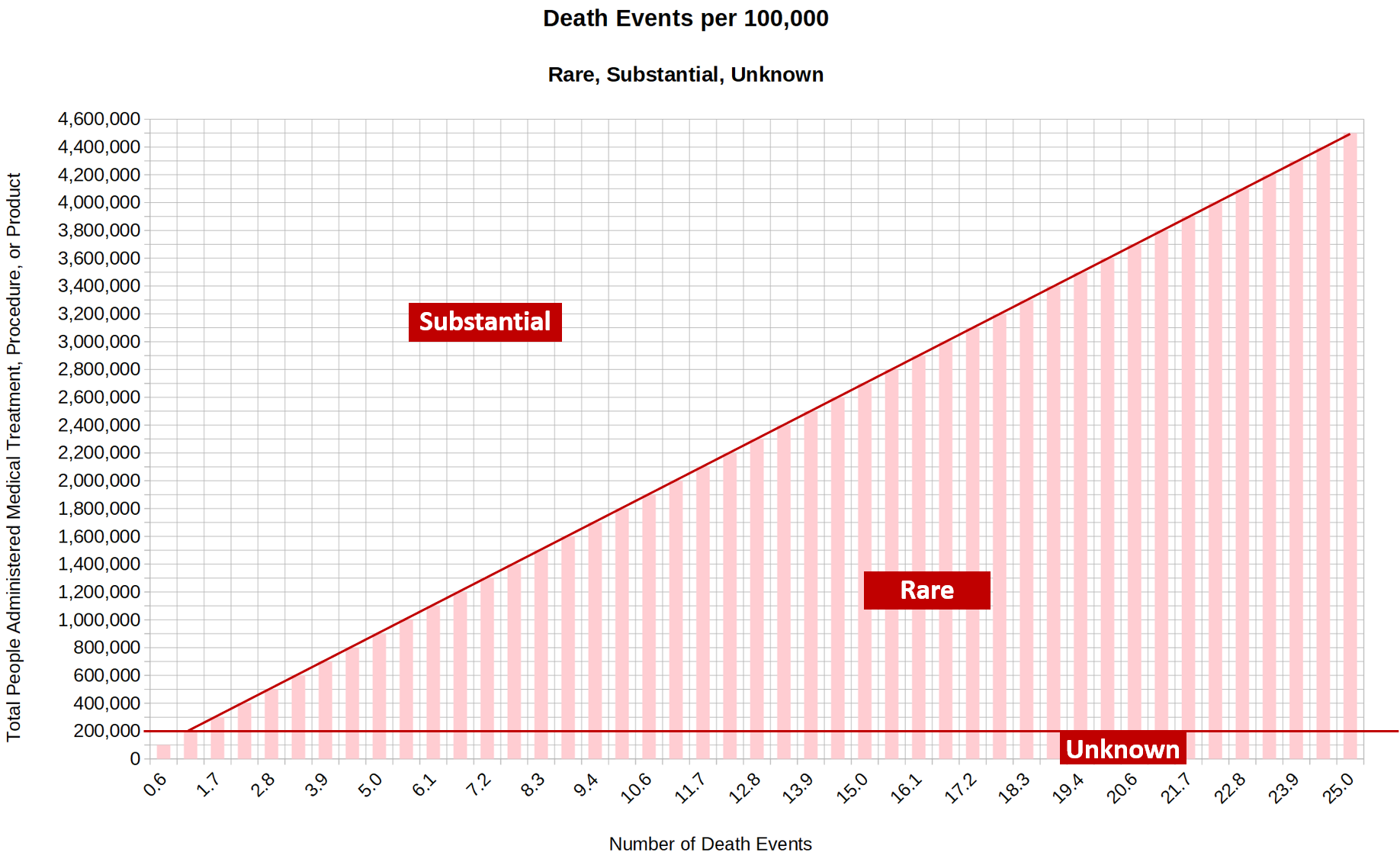
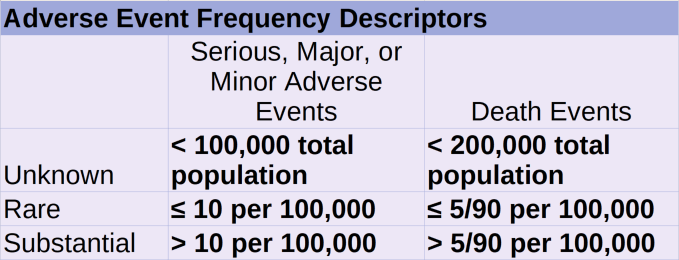
6.6.3. Adverse Event Frequency Descriptors
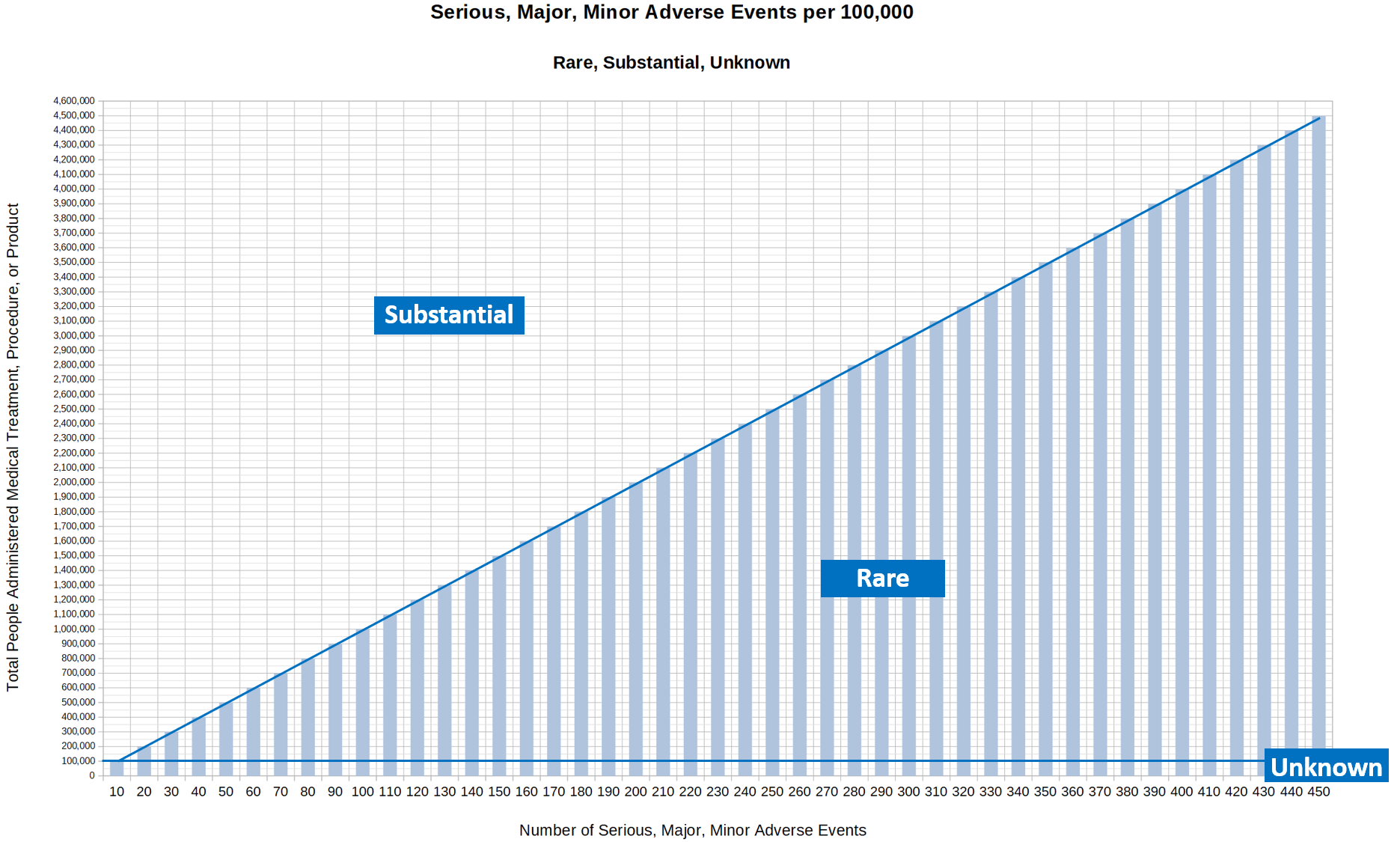
6.6.3.1. Rare
6.6.3.1.1. For Death Events
6.6.3.1.1.1. <= 5/90 per 100,000 people.
6.6.3.1.2. For Serious, Major, or Minor Events
6.6.3.1.2.1. <= 10 per 100,000 people.
6.6.3.2. Substantial
6.6.3.2.1. For Death Events
6.6.3.2.1.1. > 5/90 per 100,000 people.
6.6.3.2.2. For Serious, Major, or Minor Events
6.6.3.2.2.1. > 10 per 100,000 people.
6.6.3.3. Unknown.
6.6.3.3.1. For Death Events
6.6.3.3.1.1. Any number of death events experienced in a total treated population size of less than 200,000.
6.6.3.3.2. For Serious, Major, or Minor Events
6.6.3.3.2.1. Any number of events experienced in a total treated population size of less than 100,000.
6.7. [TRIAL] Trial Domains. A treatment or medical product may be considered conditionally safe by trial for a specific person only if that person fully shares common characteristics with the trial’s study population. Trial domains include: age, sex, weight, BMI, genetic predispositions, drug interactions, vaccination history.
6.8. [DISCLOSE] Disclosure and Advertisement.
6.8.1. The advertisement or education of a medical treatment, product, or procedure must display the existence of safety envelope information in a prominent way such that the average person may reasonably understand the product is safe, possibly safe, or experimental only under specific conditions.
6.8.2. The existence of safety envelope data must be prominently displayed on any product packaging, insert, marketing materials, or communications involving the medical treatment, procedure, or product.
6.8.3. The manufacturer, distributor, reseller, or merchant shall ensure all safety envelope data will be available to any person at any time, without obstacles to retrieval.
6.8.4. All safety envelope data will be permanently archived and permanently available to the public available to any person at any time, without obstacle, from either CDC or FDA information systems.
6.8.4.1. Requring the filing a FOIA request constitutes an obstacle.
6.9. [CLINICAL] Reporting of Clinical Trial Deficiencies
6.9.1. Any corporate entity (manufacturer) performing clinical trials shall report abnormalities to the FDA within 24 hours of an incident.
6.9.1.1. It is a felony for any employee to fail to report a trial deficiency.
6.9.1.1.1. It is a felony for any employer to act with retribution towards any employee for making this report.
6.9.1.2. The corporate entity must make it known to every employee that clinical trial deficiencies are a serious problem requiring any observing employee to report to the FDA within 24 hours via the FDA clinical trials hotline.
6.9.1.3.
6.9.1.4. The manufacturer shall retain all documents, messages, electronic messages, recordings, and data related to the trial for immediate release to FDA investigators when requested. The manufacturer shall retain all such records for a period of no less than 30 years.
6.9.2. Clinical trial deficiencies include, but shall not be limited to:
6.9.2.1. Lack of monitoring of participants.
6.9.2.2. Lack of timely follow up of any participant experiencing any degree of adverse event.
6.9.2.3. Deviations of protocol.
6.9.2.4. Purposeful or believed distortion or falsification of trial data.
6.9.2.5. Data integrity issues.
6.9.2.6. Improper storage of vaccines.
6.9.2.7. Poor laboratory management.
6.9.2.8. Mislabeled specimens.
6.9.2.9. Unblinded patients.
6.9.2.10. Inadequately trained staff.
6.9.2.11. Inadequate numbers of staff required to properly administer scientific functions.
6.9.2.12. Quality control problems.
6.9.2.13. The belief by any employee that an employer is targeting them as a result of the reporting of clinical trial deficiencies.
6.9.2.14. Lack of informed consent given to trial participant.
6.9.2.15. Any activity that is unsafe, exposes the trial participant to any danger to human life, or a condition allowed to progress which is not provided through informed consent.
6.9.2.16. Not terminating any activity which causes harm to a participant.
6.9.2.17. A lack of FDA investigatory or regulatory activity perceived by any employee.
6.9.3. It is a felony for any employee of a government or for any corporate entity to disclose the name of any person having had made a clinical trial deficiency report.
6.9.4. No employer shall discriminate or include any past activities of a person making a clinical trial deficiency report as an element of a hiring or continued employment decision.
6.10. [REAL] Reporting of Real World Events
6.10.1. No corporation shall suppress, delete, disrupt, or ask to disrupt any reporting by employees or other subordinate entities involving a real world event believed to involve a medical product.
6.10.2. Aviation
6.10.2.1. A company operating aircraft shall not suppress, delete, disrupt, or ask to disrupt any ordinary reporting or archiving of flight characteristic data such as RADAR data, ADS-B data, voice recordings, or ATC recordings from other third parties. A third party aggregating such data may not remove flight data, for any exceptional reason, from their public service where it is ordinarily making that information available for other flights.
6.10.2.2. The FAA shall maintain a de-identified database of any medications, treatments, vaccinations, or technologies used for medical purposes, for a mishap crewmember or passenger, available to the general public. A mishap is defined as: experiencing any adverse event of any severity, an incapacitation of any severity, an incident causing an individual to abandon the responsibilities of their duty position, and an excursion from the planned flight progress such as a diversion.
v.97
