Medical Freedom Act (MFA) is based on real world historical events. This button shows you historical news articles and videos that are related to part of the MFA. This lets you see for yourself why MFA is needed urgently.
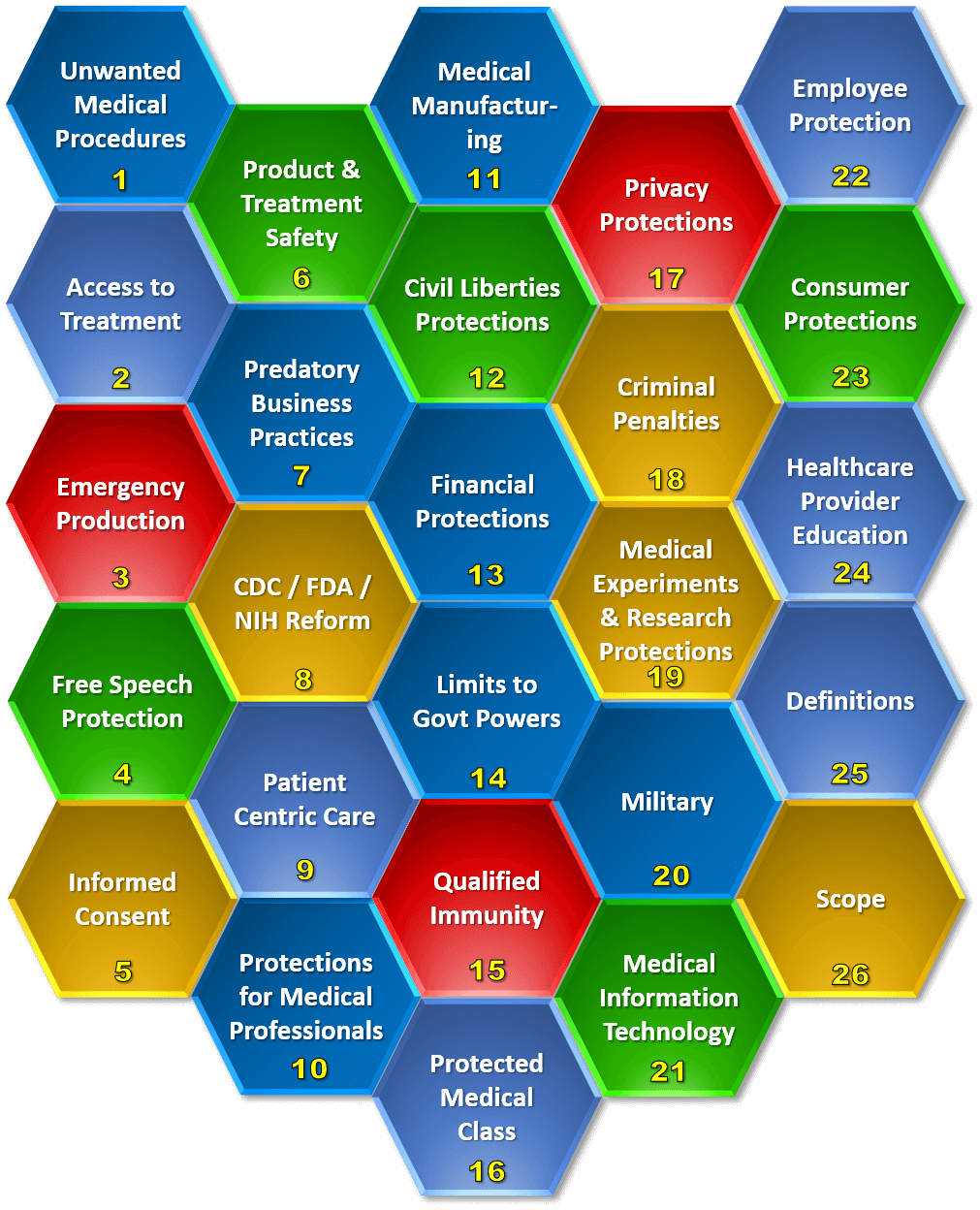
Medical Freedom Act (MFA) is based on real world historical events. This button shows you historical news articles and videos that are related to part of the MFA. This lets you see for yourself why MFA is needed urgently.
